Professor of Transfusion Medicine
Description of research
Adult stem cells reside in specialised niches that allow them to self-renew, proliferate, differentiate, and migrate according to the organism’s requirements. These niches were first discovered and are best understood in the blood system. Using the mouse bone marrow as a model, our group studies multisystem regulatory mechanisms by which the stem cell niche fulfils these complex functions and how the deregulation of these mechanisms contributes to disease. This will potentially offer novel therapeutic approaches.
Blood cells are produced in the bone marrow, which contains two distinct adult stem cell types: blood (‘haematopoietic’) stem cells (HSCs), that generate all blood and immune cells, and mesenchymal stem cells (bone marrow stem cells BMSCs), thought to form the skeleton. Our work has found a connection between the bone marrow stem-cell niche to remote signals from hormones and the brain, which can control BMSCs and HSCs through nerves that penetrate the bones associated with blood vessels. Using a multidisciplinary approach going from development to disease, we aim to understand how different types of nerves, blood vessels and BMSCs associated with them can control HSC function in health and disease. This could help find ways to obtain more stem cells, improve HSC cell transplantation procedures and treat incurable myeloproliferative disorders.
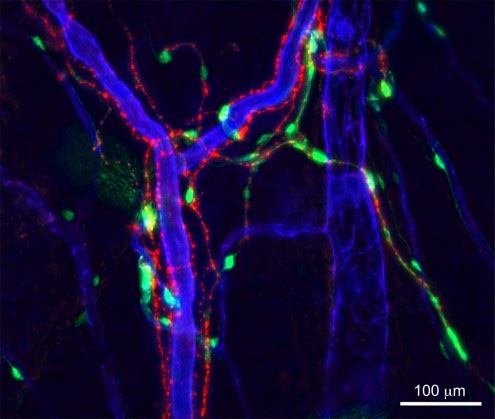
Figure 1
How niches develop and remodel in disease
The ancestry and the functional relationships among non-haematopoietic cells has remained largely unclear, in contrast to what is known about their haematopoietic neighbours in the bone marrow. We have shown that nestin+ BMSCs innervated by sympathetic nerve fibres (Figure 1) regulate normal HSCs, revealing a close interaction between both marrow stem cell types. This is also one of the first examples of organismal control of a peripheral stem cell niche through a master regulator of vertebrates − the autonomic nervous system. This neural-BMSC network might build upon shared ancestry of its cellular components, since our recent data shows that the neural crest (the source of peripheral neurons and associated Schwann cells) also contributes during development to BMSCs with a specialised HSC niche function. Dissecting how cell fate and HSC supporting properties are determined in these cells might facilitate regenerative approaches. Also, understanding these properties might facilitate finding ways to expand HSCs ex vivo. Moreover, this developmental plan might be reactivated (reprogrammed) in certain haematological diseases, and its disentangling might offer new therapeutic strategies. We are dissecting how different neurovascular beds control HSC function and their relevance in blood diseases.
Migration of blood stem cells
HSCs continuously migrate between the bone marrow and the bloodstream. This normal traffic can be intensified, allowing for the mobilisation and non-invasive harvest of HSCs for life-saving transplantation procedures. HSC transplantation is routinely performed in patients with blood cancer or inherited metabolic/immune disorders. The proper engraftment of HSCs in the bone marrow after their infusion is also critical for the transplantation’s success. Unfortunately, a number of patients are poor mobilisers and/or exhibit insufficient engraftment after transplantation.
Our recent work has determined that the number of HSCs in the blood is not constant or random, but follows instead rhythmic (circadian) oscillations governed by the molecular clock. There are two to three times more HSCs circulating in the blood during the resting period, both in mice and humans. These differences are maintained even after enforced HSC mobilisation induced by the cytokine granuylocyte colony-stimulating factor (G-CSF), commonly used in clinics. We took advantage of these oscillations to study the mechanisms regulating HSC traffic. Oscillations in HSCs mirror fluctuations in the expression of CXCL12 in the bone marrow. This chemokine is the only one known to be capable of directing HSC migration. Sympathetic nerve fibres transmit signals from the brain to the bone marrow, and control the expression of CXCL12. HSCs and leukocytes are preferentially released in the bloodstream during the resting phase and preferentially home back to the bone marrow during the activity phase. We are now investigating new pathways that control HSC migration, with the aim of improving the efficacy of HSC transplantation procedures.
Targeting the HSC niche to control disease – a novel approach
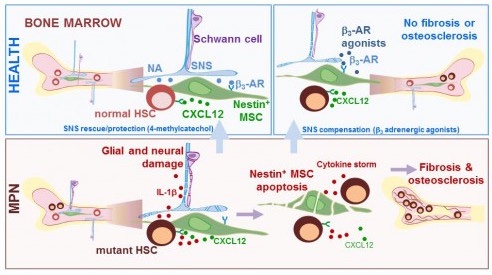
In specific blood disorders known as myeloproliferative neoplasms (MPNs), a defective gene causes blood stem cells to multiply thereby making too many. As the blood cells build up, the disease worsens, sometimes developing into cancer. Relating this fact directly to the “niche”, our team discovered that the excess mutant cells directly damage nearby nerves and MSCs, which prevent them from acting as they should in a healthy bone marrow, where they normally help to control the number and retention of blood stem cells. In this state, and left unchecked, the increase of mutant blood stem cells brings with it the likelihood of the blood disorders (MPNs). However, the disease can be blocked in mice by providing drugs which mimic the effects of the nerves (Figure 2), an approach currently being tested in a phase-II clinical study (https://clinicaltrials.gov/ct2/show/NCT02311569).
Figure 2
Hormonal regulation of bone marrow stem cells
It is likely that a combined therapy targeting both stem and niche cells will in the future be the way leukaemic stem cells can be eradicated. We are investigating new ways to target leukaemic stem cells based on susceptibility factors. One factor might be gender. For some time it has been known that men are more susceptible to leukaemias than women. We have taken this observation a step forward and found that sex hormones might explain gender differences in blood cancer susceptibility and might offer complementary ways to treat these disorders. This is because the female sex hormones oestrogens can regulate the survival, growth and maintenance of normal and mutated HSCs. We found that drugs mimicking the effects of these sex hormones can block the development of myeloproliferative neoplasms in mice, an approach that will be soon tested in humans. A phase-II clinical study will also help us understand the correlation of the disease course and in turn its response to treatment with genetic and epigenetic studies which will be mainly focused on oestrogen receptor signalling.
The importance of the stem cell niche
Overall, our research is focused on having a better understanding of how bone marrow stem cell niches work as this will potentially reveal better therapeutic targets in performing successful HSC transplants and treating currently incurable myeloproliferative diseases.
Key Publications
Méndez-Ferrer S. An AI model of transplantation risk for myelofibrosis. Blood. 2025 Jun 26;145(26):3068-3069.
Caduc MJ, de Toledo MAS, Koschmieder S, Méndez-Ferrer S. Stem Cell Niche: iPSC-Based Assembloids for Modeling Human Hematopoiesis. Methods Mol Biol. 2025 Jun 4
Khatib-Massalha E, Di Buduo CA, Chédeville AL, Ho YH, Zhu Y, Grockowiak E, Date Y, Khuat LT, Fang Z, Quesada-Salas J, Carrillo Félez E, Migliavacca M, Montero I, Pérez-Simón JA, Balduini A, Méndez-Ferrer S. Defective neutrophil clearance in JAK2V617F myeloproliferative neoplasms drives myelofibrosis via immune checkpoint CD24. Blood. 2025 May 15:blood.2024027455.
Chédeville AL, Méndez-Ferrer S. NO-immune privilege for hematopoietic stem cells. Cell Res. 2025 Feb 28.
Lisi-Vega LE, Pievani A, García-Fernández M, Forte D, Williams TL, Serafini M, Méndez-Ferrer S. Bone marrow mesenchymal stromal cells support translation in refractory acute myeloid leukemia. Cell Rep. 2025 Jan 28;44(1):115151.
Rai S, Zhang Y, Grockowiak E, Kimmerlin Q, Hansen N, Stoll CB, Usart M, Luque Paz D, Hao-Shen H, Zhu Y, Roux J, Bader MS, Dirnhofer S, Farady CJ, Schroeder T, Méndez-Ferrer S, Skoda RC. IL-1β promotes MPN disease initiation by favoring early clonal expansion of JAK2-mutant hematopoietic stem cells. Blood Adv. 2024 Mar 12;8(5):1234-1249.
Fang Z, Corbizi Fattori G, McKerrell T, Boucher RH, Jackson A, Fletcher RS, Forte D, Martin JE, Fox S, Roberts J, Glover R, Harris E, Bridges HR, Grassi L, Rodriguez-Meira A, Mead AJ, Knapper S, Ewing J, Butt NM, Jain M, Francis S, Clark FJ, Coppell J, McMullin MF, Wadelin F, Narayanan S, Milojkovic D, Drummond MW, Sekhar M, ElDaly H, Hirst J, Paramor M, Baxter EJ, Godfrey AL, Harrison CN, Méndez-Ferrer S. Tamoxifen for the treatment of myeloproliferative neoplasms: A Phase II clinical trial and exploratory analysis. Nat Commun. 2023 Nov 25;14(1):7725.
Lisi-Vega LE, Méndez-Ferrer S. The Inflamed Niche: A Double-Edged Sword in AML? Blood Cancer Discov. 2023 Sep 1;4(5):349-351
Grockowiak E, Korn C, Rak J, Lysenko V, Hallou A, Panvini FM, Williams M, Fielding C, Fang Z, Khatib-Massalha E, García-García A, Li J, Khorshed RA, González-Antón S, Baxter EJ, Kusumbe A, Wilkins BS, Green A, Simons BD, Harrison CN, Green AR, Lo Celso C, Theocharides APA, Méndez-Ferrer S. Different niches for stem cells carrying the same oncogenic driver affect pathogenesis and therapy response in myeloproliferative neoplasms. Nat Cancer. 2023 Aug;4(8):1193-1209.
Rai S, Grockowiak E, Hansen N, Luque Paz D, Stoll CB, Hao-Shen H, Mild-Schneider G, Dirnhofer S, Farady CJ, Méndez-Ferrer S, Skoda RC. Inhibition of interleukin-1β reduces myelofibrosis and osteosclerosis in mice with JAK2-V617F driven myeloproliferative neoplasm. Nat Commun. 2022 Sep 13;13(1):5346.
Chandrakanthan V, Rorimpandey P, Zanini F, Chacon D, Olivier J, Joshi S, Kang YC, Knezevic K, Huang Y, Qiao Q, Oliver RA, Unnikrishnan A, Carter DR, Lee B, Brownlee C, Power C, Brink R, Mendez-Ferrer S, Enikolopov G, Walsh W, Göttgens B, Taoudi S, Beck D, Pimanda JE. Mesoderm-derived PDGFRA+ cells regulate the emergence of hematopoietic stem cells in the dorsal aorta. Nat Cell Biol. 2022 Aug;24(8):1211-1225.
The bone marrow niche regulates redox and energy balance in MLL::AF9 leukemia stem cells. Leukemia. 2022 May 26. doi: 10.1038/s41375-022-01601-5.
Hemasphere. 2022 Apr 29;6(5):e0714. doi: 10.1097/HS9.0000000000000714.
Gadomski S, Fielding C, García-García A, Korn C, Kapeni C, Ashraf S, Villadiego J, del Toro R, Domingues O, Skepper JN, Michel T, Zimmer J, Sendtner R, Dillon S, Poole K, Holdsworth G, Sendtner M, Toledo-Aral JJ, De Bari C, McCaskie AW, Robey RG, Méndez-Ferrer S. A cholinergic neuroskeletal interface promotes bone formation during postnatal growth and exercise. Cell Stem Cell 29, 2022; doi: 10.1016/j.stem.2022.02.008.
Fielding C, García-García A, Korn C, Gadomski S, Fang Z, Reguera JL, Pérez-Simón JA, Göttgens B, Méndez-Ferrer S. Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis. Nat Commun. 2022 Jan 27;13(1):543. doi: 10.1038/s41467-022-28175-1.
Valet C, Magnen M, Qiu L, Cleary SJ, Wang KM, Ranucci S, Grockowiak E, Boudra R, Conrad C, Seo Y, Calabrese DR, Greenland JR, Leavitt AD, Passegué E, Méndez-Ferrer S, Swirski FK, Looney MR. Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression. J Clin Invest 2022 Feb 22;e153920. doi: 10.1172/JCI153920.
Prados Pinto B, del Toro R, MacGrogan D, Gómez-Apiñániz P, Papoutsi T, Munoz-Canoves P, Méndez-Ferrer S, Jose Luis de la Pompa. Heterotopic ossification in mice overexpressing Bmp2 in Tie2+ lineages. Cell Death & Disease 2021 Jul 22;12(8):729. doi: 10.1038/s41419-021-04003-0ress.
Renders S, Svendsen AF, Panten J, Rama N, Maryanovich M, Sommerkamp P, Ladel L, Redavid AR, Gibert B, Lazare S, Ducarouge B, Schönberger K, Narr A, Tourbez M, Dethmers-Ausema B, Zwart E, Hotz-Wagenblatt A, Zhang D, Korn C, Zeisberger P, Przybylla A, Sohn M, Méndez-Ferrer S, Heikenwälder M, Brune M, Klimmeck D, Bystrykh L, Frenette PS, Mehlen P, de Haan G, Cabezas-Wallscheid N, Trumpp A. Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1. Nat Commun. 2021 Jan 27;12(1):608.
Forte D, García-Fernández M, Sánchez-Aguilera A, Stavropoulou V, Fielding C, Martín-Pérez D, López JA, Costa ASH, Tronci L, Nikitopoulou E, Barber M, Gallipoli P, Marando L, Fernández de Castillejo CL, Tzankov A, Dietmann S, Cavo M, Catani L, Curti A, Vázquez J, Frezza C, Huntly BJ, Schwaller J, Méndez-Ferrer S. Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab. 2020 Sep 16:S1550-4131(20)30479-4.
Méndez-Ferrer S, Bonnet D, Steensma DP, Hasserjian RP, Ghobrial IM, Gribben JG, Andreeff M, Krause DS. Bone marrow niches in haematological malignancies. Nat Rev Cancer. 2020 May;20(5):285-298.
Ho YH, Méndez-Ferrer S. Microenvironmental contributions to hematopoietic stem cell aging. Haematologica. 2020 Jan;105(1):38-46. doi: 10.3324/haematol.2018.211334. Epub 2019 Dec 5.
Ho YH, Del Toro R, Rivera-Torres J, Rak J, Korn C, García-García A, Macías D, González-Gómez C, Del Monte A, Wittner M, Waller AK, Foster HR, López-Otín C, Johnson RS, Nerlov C, Ghevaert C, Vainchenker W, Louache F, Andrés V, Méndez-Ferrer S. Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging. Cell Stem Cell. 2019 Sep 5;25(3):407-418.e6
Saçma M, Pospiech J, Bogeska R, de Back W, Mallm JP, Sakk V, Soller K, Marka G, Vollmer A, Karns R, Cabezas-Wallscheid N, Trumpp A, Méndez-Ferrer S, Milsom MD, Mulaw MA, Geiger H, Florian MC. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol. 2019 Nov;21(11):1309-1320.
Drexler B, Passweg JR, Tzankov A, Bigler M, Theocharides AP, Cantoni N, Keller P, Stussi G, Ruefer A, Benz R, Favre G, Lundberg P, Nienhold R, Fuhrer A, Biaggi C, Manz MG, Bargetzi M, Méndez-Ferrer S, Skoda RC; Swiss Group for Clinical Cancer Research (SAKK). The sympathomimetic agonist mirabegron did not lower JAK2-V617F allele burden, but restored nestin-positive cells and reduced reticulin fibrosis in patients with myeloproliferative neoplasms: results of phase II study SAKK 33/14. Haematologica. 2019 Apr;104(4):710-716.
García-García A, Korn C, García-Fernández M, Domingues O, Villadiego J, Martín-Pérez D, Isern J, Bejarano-García JA, Zimmer J, Pérez-Simón JA, Toledo-Aral JJ, Michel T, Airaksinen MS, Méndez-Ferrer S. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood. 2019 Jan 17;133(3):224-236.
Mangolini M, Götte F, Moore A, Ammon T, Oelsner M, Lutzny-Geier G, Klein-Hitpass L, Williamson JC, Lehner PJ, Dürig J, Möllmann M, Rásó-Barnett L, Hughes K, Santoro A, Méndez-Ferrer S, Oostendorp RAJ, Zimber-Strobl U, Peschel C, Hodson DJ, Schmidt-Supprian M, Ringshausen I. Notch2 controls non-autonomous Wnt-signalling in chronic lymphocytic leukaemia. Nat Commun. 2018 Sep 21;9(1):3839.
Golan K, Kumari A, Kollet O, Khatib-Massalha E, Subramaniam MD, Ferreira ZS, Avemaria F, Rzeszotek S, García-García A, Xie S, Flores-Figueroa E, Gur-Cohen S, Itkin T, Ludin-Tal A, Massalha H, Bernshtein B, Ciechanowicz AK, Brandis A, Mehlman T, Bhattacharya S, Bertagna M, Cheng H, Petrovich-Kopitman E, Janus T, Kaushansky N, Cheng T, Sagi I, Ratajczak MZ, Méndez-Ferrer S, Dick JE, Markus RP, Lapidot T. Daily Onset of Light and Darkness Differentially Controls Hematopoietic Stem Cell Differentiation and Maintenance. Cell Stem Cell. 2018 Oct 4;23(4):572-585.e7.
Merlo A, Bernardo-Castiñeira C, Sáenz-de-Santa-María I, Pitiot AS, Balbín M, Astudillo A, Valdés N, Scola B, Del Toro R, Méndez-Ferrer S, Piruat JI, Suarez C, Chiara MD. Role of VHL, HIF1A and SDH on the expression of miR-210: Implications for tumoral pseudo-hypoxic fate. Oncotarget. 2017 Jan 24;8(4):6700-6717. doi: 10.18632/oncotarget.14265 (2016).
Del Toro R, Chèvre R, Rodríguez C, Ordóñez A, Martínez-González J, Andrés V, Méndez-Ferrer S. Nestin(+) cells direct inflammatory cell migration in atherosclerosis. Nat Commun. 2016 Sep 2;7:12706. doi: 10.1038/ncomms12706.
Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntión S, Tzeng YS, Lai DM, Schwaller J, Skoda RC, Méndez-Ferrer S. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature512, 78-81, doi:10.1038/nature13383 (2014).
Sánchez-Aguilera A, Arranz L, Martín-Pérez D, García-García A, Stavropoulou V, Kubovcakova L, Isern J, Martín-Salamanca S, Langa X, Skoda RC, Schwaller J, Méndez-Ferrer S. Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell stem cell 15, 791-804, doi:10.1016/j.stem.2014.11.002 (2014).
Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F, Méndez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem-cell-niche function. eLife 3, doi:10.7554/eLife.03696 (2014).
Isern, J, Martín-Antonio B, Ghazanfari R, Martín AM, López JA, del Toro R, Sánchez-Aguilera A, Arranz L, Martín-Pérez D, Suárez-Lledó M, Marín P, Van Pel M, Fibbe WE, Vázquez J, Scheding S, Urbano-Ispizúa Á, Méndez-Ferrer S. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep, 1714-1724, doi:10.1016/j.celrep.2013.03.041 (2013).
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829-834, doi:10.1038/nature09262 (2010).
Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442-447, doi:10.1038/nature06685 (2008).